
O–H stretch, hydrogen bonded 3500-3200 cm -1įigure 7.Infrared Spectrum of Tolueneįunctional Groups Containing the C-O BondĪlcohols have IR absorptions associated with both the O-H and the C-O stretching vibrations. Only alkenes and aromatics show a C–H stretch slightly higher than 3000 cm -1.įigure 6. This is a very useful tool for interpreting IR spectra. Note that this is at slightly higher frequency than is the –C–H stretch in alkanes. In aromatic compounds, each band in the spectrum can be assigned:
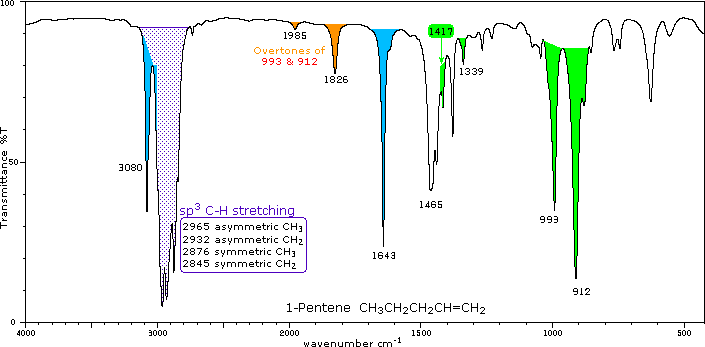
The spectrum of 1-hexyne, a terminal alkyne, is shown below. In alkynes, each band in the spectrum can be assigned: As alkanes compounds, these bands are not specific and are generally not noted because they are present in almost all organic molecules. In alkenes compounds, each band in the spectrum can be assigned:įigure 4. Note that the change in dipole moment with respect to distance for the C-H stretching is greater than that for others shown, which is why the C-H stretch band is the more intense. Since most organic compounds have these features, these C-H vibrations are usually not noted when interpreting a routine IR spectrum. C–H rock, methyl, seen only in long chain alkanes, from 725-720 cm -1įigure 3. Not specified, most likely a prism, grating, or hybrid spectrometer.C–H bend or scissoring from 1470-1450 cm -1.In alkanes, which have very few bands, each band in the spectrum can be assigned: Hydrocarbons compounds contain only C-H and C-C bonds, but there is plenty of information to be obtained from the infrared spectra arising from C-H stretching and C-H bending.


 0 kommentar(er)
0 kommentar(er)
